Delaying Type 1 Diabetes Progression
If you have high blood pressure, treating it can delay or prevent damage that could lead to heart disease. That’s the idea behind delaying the development of stage 3 T1D, which can1:
Reduce complications.
The longer you have T1D, the greater your risk for complications including heart and kidney disease nerve damage, and vision problems. Delaying progression can help reduce the risk and severity of these complications.

Reduce medical costs.
Healthcare is expensive, especially for a complex chronic condition such as T1D. Delaying progression means fewer drugs, doctor visits, hospitalizations, and tests—all of which can help reduce your out-of-pocket costs.
Improve your quality of life.
It is easier to manage T1D in the earlier stages when your body is still making some insulin; this, in turn, lets you live a more normal life without intensive medical treatments.

Provide an opportunity for new therapies.
Numerous clinical trials are testing new drugs designed to delay, reverse, or even prevent the development of T1D. Remaining in the early stages of T1D makes you eligible for these studies. In addition, you will be able to try those drugs that are approved.

Preserve pancreatic function.
As you recall, specialized cells in the pancreas called beta cells make insulin. In the early stages of T1D, some of those cells are still working. Delaying progression may preserve their function, improving blood sugar control and reducing the need for insulin therapy.

Improve your mental health.
Managing a chronic condition such as diabetes can take a toll on mental health. Delaying progression can ease some of the stress and anxiety associated with the disease.
New Drugs That Delay T1D Progression
In 2022, the US Food and Drug Administration approved the first drug to delay progression to stage 3 of T1D in people 8 years old and older who are already in stage 2. You are in stage 2 T1D if you tested positive for 2 or more T1D-related autoantibodies, have abnormal blood sugar levels, and no symptoms.2
Of course, the only way to know if you are in stage 2 is by getting screened.
The drug is called teplizumab. It’s an antibody, which is a type of protein that can recognize and attach to a specific receptor on the surface of immune cells called T cells. Think of it as inserting a key into a lock. This helps “calm” those cells, so they back off from destroying pancreatic beta cells. In turn, those beta cells maintain the ability to produce insulin, delaying the development of full-blown T1D.3
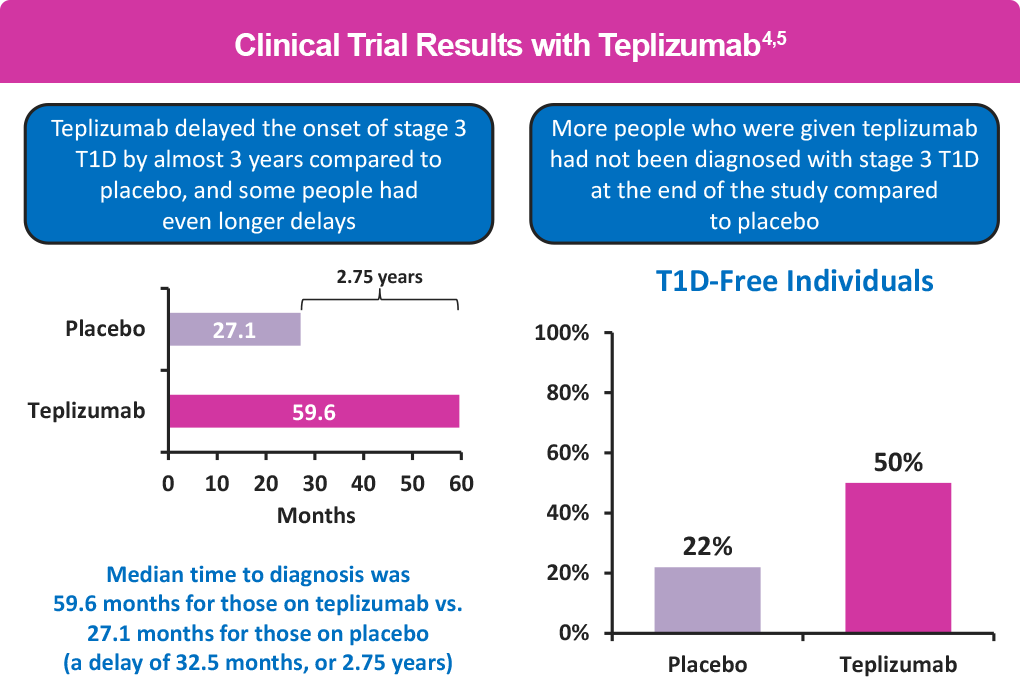
The FDA approved teplizumab based on a study called AT-Risk. The study enrolled relatives of people with T1D who had a high risk of developing the disease. After 6 years more than half (55%) of those receiving teplizumab were diabetes free vs about 22% of the people receiving a placebo. Teplizumab delayed the onset of stage 3 by more than 2 years (32.5 months) compared with placebo, with longer delays in some people.4, 5
Side effects of teplizumab are mild to moderate. The most common side effects seen in the studies were rash, headache, and low white blood cell counts.4,5 Very rarely, a condition called cytokine release syndrome (CRS) may occur. In CRS, your immune system responds to the drug more aggressively than it should. Symptoms include fever, nausea, fatigue, and body aches. Tell your doctor immediately if you experience any of these symptoms while taking teplizumab.6
You receive teplizumab in an infusion center every day for 14 days. A half hour before the infusion, you’ll receive an antihistamine and an anti-nausea drug to reduce the risk of side effects. Then the infusion nurse will insert an intravenous (IV) medication into your arm, allowing it to flow directly into your veins. It takes about 30 minutes for each infusion.2 For people who prefer not to get an IV every day for 2 weeks, particularly children, the doctor may implant a central port, also known as a central venous access port (or implanted venous access device) a few days before you start treatment. This is a tube and reservoir that’s inserted under the skin to access a vein so you don’t have be stuck with a needle for each treatment7
You’ll have blood work done before you start on teplizumab and during treatment to check for any signs of liver damage or changes to your white blood cells and to monitor for infections.2
Clinical trials that are testing numerous other treatments that work on the immune system are underway. These include drugs to stem immune cell attacks on beta cells, reduce inflammation, and protect beta cell function. You may be eligible to participate in those trials. Links can be found
In the Resources section of this web-site.
Questions to Ask Your Doctor About Teplizumab Treatment
- Is my child (or me) eligible for teplizumab?
- Does the infusion hurt?
- Does my child have to get an IV every day? Is there an easier way?
- Will my insurance cover treatment?
- What side effects should I expect?
- When should I call you about side effects?
- Is there any way to reduce the risk of side effects?
- What are the chances it will work?
- What if it doesn’t work?
References
- University of Colorado – Barbara Davis Center for Diabetes. Ask the experts for early T1D answers and guidance – T1D information for families. https://www.asktheexperts.org/for-families
- Teplizumab (Tzield®). Prescribing information 2023. Provention Bio. https://products.sanofi.us/tzield/tzield.pdf
- Seewoodhary J, Silveira A. Teplizumab — preventative approaches to type 1 diabetes mellitus. Pract Diab. 2023;40:35-38a. https://wchh.onlinelibrary.wiley.com/doi/epdf/10.1002/pdi.2448
- Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381:603-613.
- Sims EK, Bundy BN, Stier K, et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med. 2021;13:eabc8980.
- National Cancer Institute (NCI). Cytokine release syndrome. www.cancer.gov/publications/dictionaries/cancer-terms/def/cytokine-release-syndrome
- Medical Central venous catheters-ports. https://medlineplus.gov/ency/patientinstructions/ 000491.htm#:~:text=A%20central%20venous%20catheter%20is,than%20just%20having%20the%20catheter